As supply and demand imbalances intensify, locum tenens work emerges as a crucial solution, offering surgeons unprecedented opportunities to capitalize on market needs.
Recent Posts
-

Top Surgical Specialties by Pay
-

Top 5 States for Travel Healthcare Positions in Summer 2025
Summer 2025 is brimming with exciting opportunities for healthcare professionals across these top five states and beyond.
-

How Locum Tenens Fits Into Modern Healthcare Staffing Solutions
Hospitals, clinics, and other facilities are already reeling from a shortage of providers and it’s hurting patients—tens of millions of Americans live in areas with shortages of primary care, dental, and mental health professionals.
-

My Locum Story: Dentist Laurence M.’s Return to Work As a Locum Provider After Selling His Private Practice
“My first experience was great,” he said. “When it was over, I called Jordan Kelly and said, ‘Okay, find me another assignment.’”
-

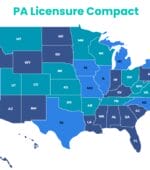
PA Licensure Compact Enacted in 19 States
A total of 19 states have enacted the PA Licensure Compact, but the agreement has yet to be operationalized.
-

2025 Doctor Job Outlook: Why Go Locum Tenens?
According to the United States Bureau of Labor Statistics (BLS), overall employment of physicians is projected to grow 4% between 2023 and 2033.
-

Why Partner with a Locum Tenens Agency for Dental Staffing?
Dental staffing solutions can lower your administrative burden and staff burnout while providing access to a wide talent pool of dentists. Read on to learn how your facility could benefit.
-

4 Ways Temporary Medical Staff Maintain Patient Care
Many healthcare facilities in the U.S. are already struggling with staffing shortages, but others have turned to healthcare staffing agencies like Barton Associates to fill in the gaps.
-

Psychiatric Mental Health Nurse Practitioner: Frequently Asked Questions (FAQs)
Behavioral health needs have climbed in the United States over the past decade—between the opioid epidemic and the COVID-19 pandemic, more and more Americans are dealing with mental health challenges.
-

Medical Staffing Solutions: 4 Key Qualities of Top Medical Staffing Agencies
We’re diving deep into the four key qualities you should look for in medical staffing agencies and explaining how locum tenens providers can help you ensure continuity of patient care.